Sepsis is one of the ten leading causes of death worldwide. According to the Centers for Disease Control, more than 1.7 million people in the United States contract sepsis yearly, and around 270,000 of these people die from its associated complications.
In 2019, sepsis added more than $62 billion in healthcare costs alone in the US, and the incidence of sepsis and associated healthcare costs continues to increase. Despite many advances in care, fully effective treatment strategies for sepsis have unfortunately remained elusive.
Pathobiology of Sepsis
Sepsis is life-threatening organ dysfunction that develops due to the dysregulated host response to uncontained microbial infection. The overall pathobiology of sepsis (Fig. 1) is complicated and involves early activation of both pro-and anti-inflammatory responses and major modifications in nonimmunologic pathways.
How sepsis affects a patient is greatly influenced by their age and general health. Potential effective therapeutics for the prevention and treatment of sepsis include antimicrobial agents, vaccine strategies for causative organisms, mitigation of the dysregulated antimicrobial host response, and palliative care.
Infectious Disease Models of Sepsis
Antimicrobial and immunomodulatory agents have the potential to perform several actions that could prevent the establishment of sepsis and mitigate the complications of the resulting dysregulated host response.
Initial identification of effective antimicrobial agents generally starts at the in vitro stage, where candidate therapeutics are evaluated for their ability to inhibit the replication of or kill a range of clinically relevant infectious agents. The in vitro characterization may also identify the mechanism of action of the prospective therapeutics and its ability to circumvent antimicrobial resistance.
Preclinical identification and testing of new therapeutics for the treatment of sepsis requires suitable animal models capable of characterizing microbial replication and the impact of the prospective therapeutic on the pathobiology of sepsis. The cascade of events leading to sepsis can be a direct effect of the systemic invasion of an infectious agent or its toxic product(s). Routes of systemic invasion include complications from peritonitis, bronchial infection and infected wounds, or direct exposure of the bloodstream to the infectious agent or its toxic products (endotoxemia), conditions that can be created in animal models of infection.
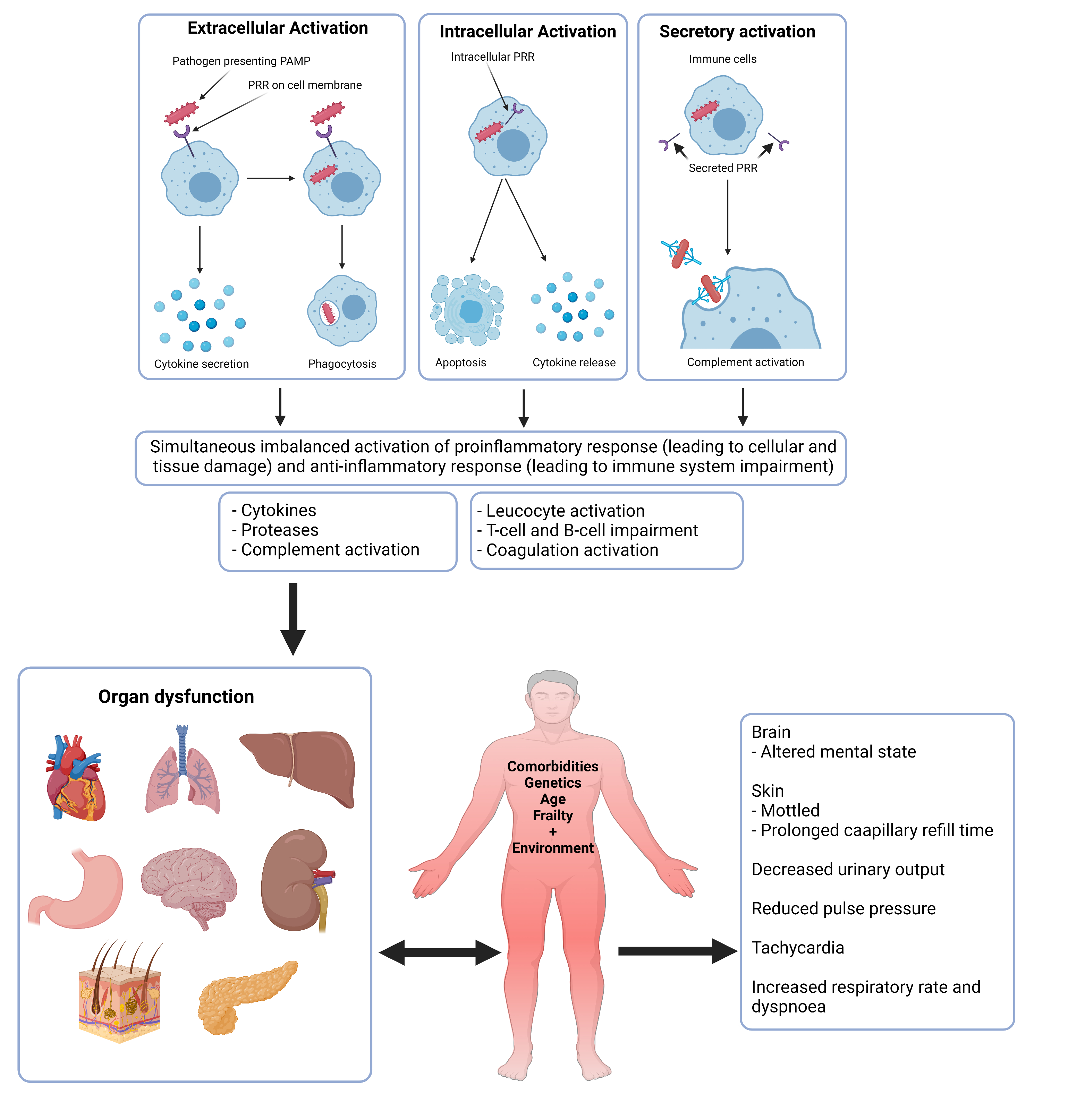
Figure 1. Pathobiology of Sepsis.
PAMP=pathogen-associated molecular pattern. PRR=pattern recognition receptor.
At Noble Life Sciences, we have established in vitro assays and animal models to evaluate bacterial sepsis. Animal models include murine peritonitis models using a single infectious organism (MRSA) or bacteremia from intestinal contents (cecal slurry) (Fig. 2). Our animal models for toxicology can be used to establish endotoxemia-induced sepsis.
These models can be performed with animals of various ages to mimic clinically relevant conditions and multiple endpoint analyses such as bacterial load, immunological biomarkers, and histology, which can be utilized to evaluate the effectiveness and mechanism of the candidate therapeutic. The breadth of experience of Noble Life Sciences’ scientific and technical staff provides the ability to modify current models or develop custom infectious disease models to meet your goals in developing therapeutics for the prevention and treatment of sepsis.
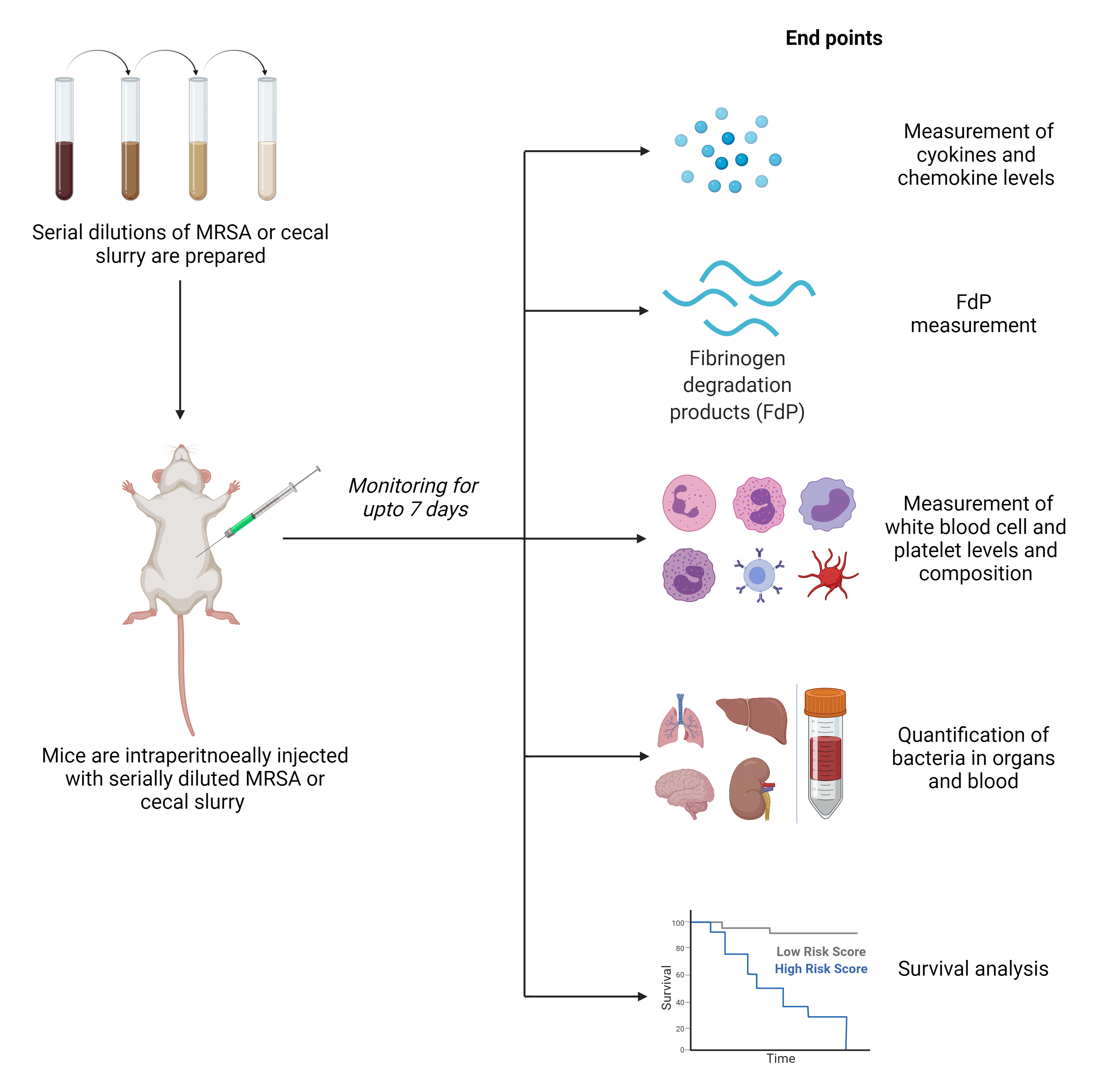
Figure 2. Schematic representation of Mouse MRSA and Cecal Slurry Sepsis Models
Multidisciplinary Approach to Preclinical Drug Development
Noble Life Sciences provides a broad range of preclinical drug discovery and development services. From in vitro analysis to animal models of infection and disease, toxicology, cell biology, molecular biology, immunology, and oncology, we can support your research and be a valuable addition to your scientific team. Our programs are designed to help you move your research from bench to bedside in an effective and timely manner.
Located in Sykesville, Noble Life Sciences is one of Maryland’s leading contract research organizations for preclinical studies. Our strategic location near Johns Hopkins University gives us an advantage in supporting numerous biotechnology companies and universities in the area.
Contact us at info@noblelifesci.com today to find out how we can help you achieve your preclinical goals.
