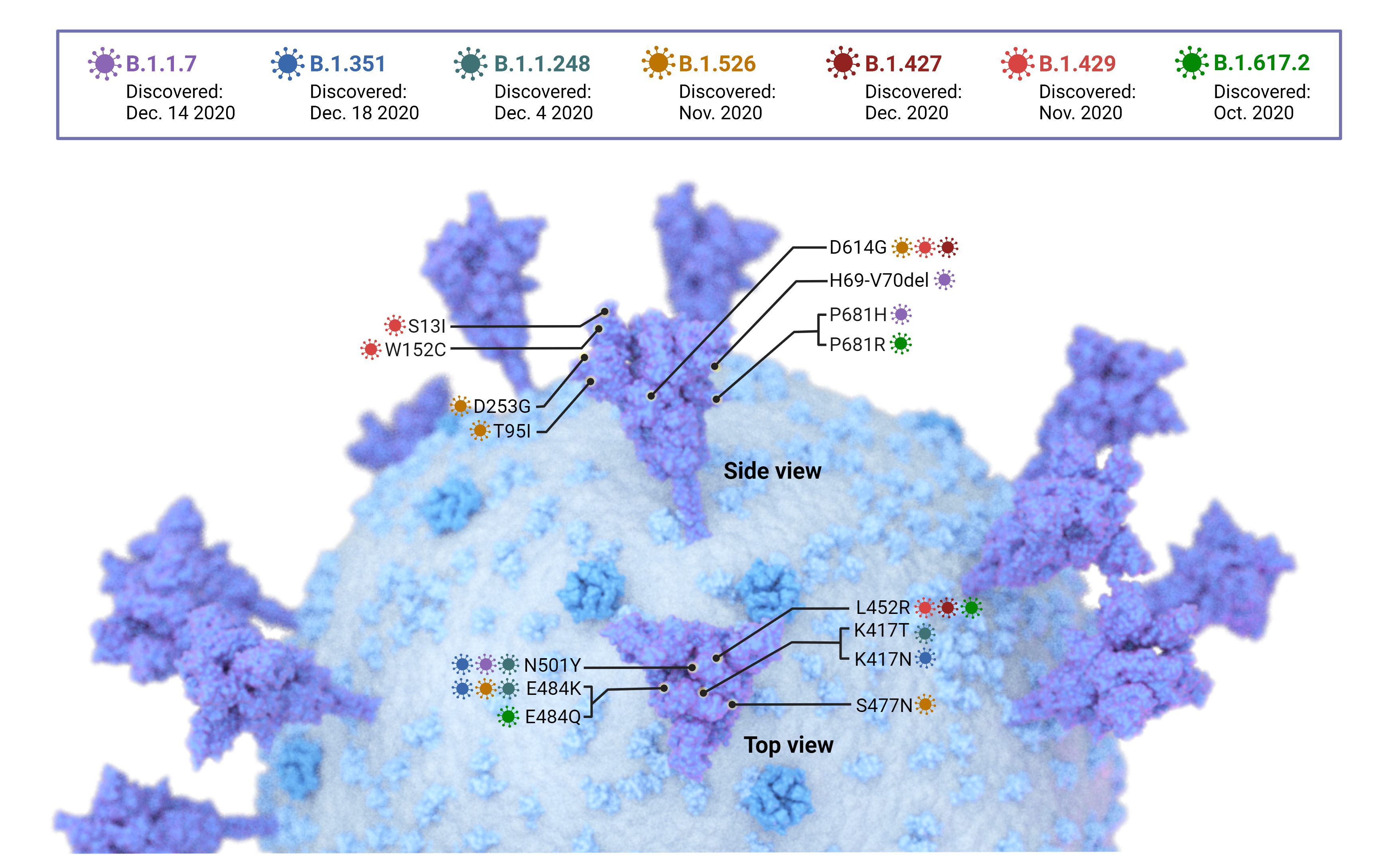
Figure 1 Key mutations in the spike protein of SARS-CoV Variants of Concern (VoC) (B.1.1.7, B.1.351, B.1.1.248, and B.1.617.2) and Variants of Interest (B.1.526, B.1.427, and B.1.429). Mutations occur on all spike protein subunits. Mutations in other areas of the genome have been identified and are currently under investigation.
Introduction
Since its emergence in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide, causing a global pandemic (coronavirus disease-19 or COVID-19) with more than 188 million confirmed infections and more than 4 million fatalities (as of July 19, 2021) (https://covid19.who.int). While the majority of the infections are asymptomatic or manifest in mild to moderate forms, a small percentage of people develop severe respiratory illness with a fatal outcome.
Since the start of the pandemic, tremendous progress has been made in the design, authorization, and deployment of SARS-CoV-2 vaccines. All the vaccines aim to prevent COVID-19 primarily by eliciting neutralizing antibodies that block the spike protein and prevent the ability of SARS-CoV-2 to infect cells. However, various SARS-CoV-2 variants are now emerging in many parts of the world, posing a threat of increased infections.
So far, four major circulating SARS-CoV-2 variants of concern (VoC) have been identified; lineages B.1.1.7 or alpha (α), B.1.351 or beta (β), B.1.1.28.1 or gamma (γ), and B.1.617.2 or delta (δ). Mutations in the spike protein (Figure 1) of these VoC are responsible for increased transmissibility, reinfection rates, and decreased vaccine effectiveness. Therefore, the development of more effective therapeutics is crucial for protection against COVID-19. Thus, animal models that closely mimic the pathogenesis of COVID-19 in humans are essential for understanding disease pathways and evaluating prospective treatments.
A new study published in EBioMedicine compared the relative infectivity and virulence of VoC B.1.1.7 and B.1.351 with the two basal SARS-CoV-2 strains, BetaCov/Belgium/GHB-03021/2020 (B.1-G) and Germany/BavPat1/2020 (B.1-B) in Syrian Golden Hamsters. Hamsters were intranasally infected with 105 TCID viral stocks and euthanized four days post-infection for further analysis. Both VoC replicated efficiently in the lower respiratory tract causing severe pathological changes in the lungs. Microcomputed tomographic (micro-CT) imaging revealed severe lung injury that shared characteristics with SARS-CoV-2−infected human lungs. Additionally, all variants significantly increased the expression levels of pro-inflammatory cytokines in lung tissues. Overall, the findings of this study demonstrate that hamsters can serve as a relevant preclinical model for assessing the infectivity of clinical SARS-CoV-2 isolates, including the current VoC. This model can also serve as a tool to evaluate current and future vaccines and antiviral therapeutics against important SARS-CoV-2 VoC.
As SARS-CoV-2 continues to spread globally, Noble Life Sciences (NLS) is committed to providing support for finding an effective immune and prophylactic strategy. Our infectious disease drug development services team delivers the highest quality, full-service contract research to support the pharmaceutical and biotechnology sectors. NLS can assist you in the preclinical drug development of SARS-CoV-2 antivirals and vaccines. Our scientists are experienced in animal model efficacy testing, and our facilities have the capability in BSL3 containment. Our facilities have been accredited fully and continuously by the Association for the Assessment and Accreditation of Laboratory Animals (AAALAC) since 1989. Noble is an FDA inspected GLP facility. The company operates in full accordance with the Animal Welfare Act, the PHS Policy on Humane Care and Use of Laboratory Animals, and the NRC Guide for the Care and Use of Laboratory Animals. In addition, NLS is approved to acquire and work with clinically relevant SARS-CoV-2 isolates.
Our services include:
- Early-stage preclinical solutions
- Established SARS-CoV-2 mouse models - hACE2 and K3 transgenic mouse models.
- Individual study design to answer specific scientific research question(s).
- Prophylactic and therapeutic efficacy testing of antivirals
- Immunization and challenge studies for vaccine efficacy.
- Immunogenicity studies
- Pharmacokinetic studies
- Virological assays
- Viral titration assays (TCID5O)
- Antiviral assays (IC50)
- Molecular assays
- qRT-PCR
- Next-generation sequencing
- Imaging services
- Live animal imaging
- Computed Tomography (CT)
- Immunological assays
- ELISA
- Flow cytometry
- Neutralizing antibody assays
- Immunoassay development
If you require any additional information or if you would like to discuss specific queries regarding your upcoming project, please feel free to contact our dedicated team at: info@noblelifesci.com
