Introduction
Immuno-oncology therapeutics, also known as cancer immunotherapy, have long been an integral part of cancer treatment. However, recent advancements have brought it to the forefront of oncology care. Various cancer immunotherapy approaches today include adoptive cell transfer, cancer vaccines, checkpoint inhibitors, and immune system modulators. However, the two principal factors behind the success of cancer immunotherapy are checkpoint inhibitors (CPIs) and chimeric antigen receptor (CAR) T-cells (a type of adoptive cell transfer).
CPIs, such as anti-PD1/PD-L1 and anti-CTLA4 antibodies, block immune checkpoint receptors on tumor cells, thereby disrupting immune inhibitory signals and restoring T-cell cytotoxicity against cancer cells. On the other hand, CAR-T-cell-based therapies involve genetic modification of patients’ T-cells to express CAR targeted at a tumor-specific antigen. CAR T-cells can then bind with cancer cells to stimulate T-cell activation and cytotoxicity against the tumor. However, despite showing great promise in a wide variety of solid and hematological malignancies, limited success has been observed with these agents in several other types of cancers.
A critical challenge in developing effective cancer immunotherapy lies in the development of relevant models that can mimic tumor microenvironment (TME) anatomically, genetically, and physiologically. High tumor growth rates and easy genetic modification allow mouse models to be valuable tools for evaluating the effectiveness of immuno-oncology therapeutics. However, translation of preclinical results to clinical efficacy is challenging because of the high attrition rate observed in oncology trials compared to other therapeutic areas. This blog discusses various preclinical mouse models used to evaluate the effectiveness of immuno-oncology therapeutics, particularly CPIs and CAR T-cells.
Syngeneic tumor models
In syngeneic tumor models (Figure 1), murine tumor cell lines are grown in vitro and then transplanted into immunocompetent hosts, such as C57BL/6, BALB/c, or FVB mice, usually subcutaneously or orthotopically. Syngeneic models are often the first experimental approach for studying immuno-oncology therapeutics because of their intact immune system, which allows studying complex interactions between immune cells and tumors. Syngeneic models also allow incorporation of a tracker, such as luciferase, or genetic manipulation to monitor immune system components both systemically and at the tumor site. In addition, these models are easy to use and exhibit fast tumor growth and high reproducibility.
Various syngeneic tumor models for different tissues are available. However, the three most popular models that provide many of the characteristics needed to evaluate immunotherapeutics include the 4T1 Breast Carcinoma Model, B16F10 Melanoma Model, and CT26 Colon Cancer Model.
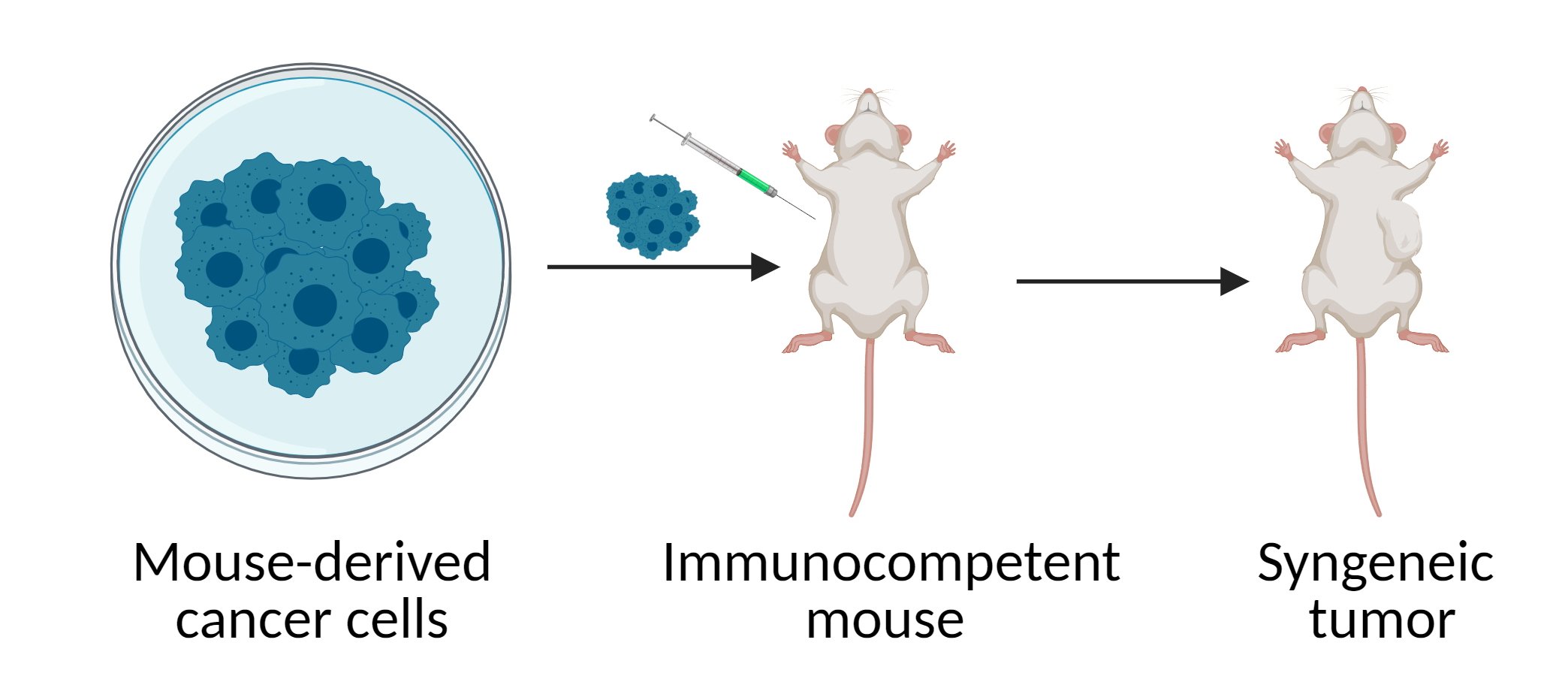
Figure 1 Syngeneic mouse model
Genetically engineered mouse models (GEMMs)
GEMMs (Figure 2) are generated through the systemic or tissue-specific expression of oncogenes, such as BRAFV600E in melanoma, and/or deletion of tumor suppressor genes, such as APC in colon cancer. GEMMs are further classified into germline and non-germline GEMMs. Germline GEMMs carry genetic modifications in the germline and allow tumors to develop spontaneously but offer little control over space and time of tumor onset. Non-germline GEMMs, on the other hand, enables induction of mutation at a specific time and tissue, which can be achieved using various systems such as CRISPR/Cas9, shRNA, and Cre-lox technologies.
Gradual de novo tumor development and progression in GEMMs closely mimic histopathological and molecular features of human cancers, display genetic heterogeneity, and progress spontaneously to metastatic disease. Consequently, GEMMs provide a more complex interaction with the immune system since the tumor passes through all immunosurveillance stages. However, an increased mutational burden in GEMMs can lead to the development of an anti-cancer immune response, influencing the effectiveness of immunotherapy. As a result, GEMMs are poorly reproducible compared to syngeneic models.
The development of GEMMs is a very time-consuming and expensive process. As the evaluation of immunotherapy in GEMMs with TME represented by mouse cells yields mixed results, their use to evaluate CPIs and CAR T-cell therapies remains limited.
F
Figure 2 Genetically Engineered Mouse Model
Patient-derived xenograft (PDX) models
In PDX models (Figure 3), the tumor fragments are surgically removed from cancer patients and are subcutaneously or orthotopically transplanted into immunocompromised hosts such as athymic nude or severe combined immunodeficiency syndrome (SCID) mice. The degree of immunodeficiency of murine host determines the utility of PDX models for immunotherapeutic applications.
PDX models precisely mirror the complexities of natural tumor development, including tumor heterogeneity, architecture, and microenvironment. PDX models generated from hematopoietic and lymphoid tissue tumors have been used to evaluate the efficacy of CAR T-cell-based therapies. It is also possible to reconstruct the immune system using PDX models to assess the effectiveness of CPIs. This is achieved by transplanting human hematopoietic stem cells (HSCs) and peripheral blood mononuclear cells (PBMCs) into immunocompromised mice bearing human leukocyte antigen (HLA)-matched human immune system (humanized mice). In recent years, PDX generated from CD34+ cells, and tumor-infiltrating lymphocytes have been recently developed.

Figure 3 Patient-derived xenograft (PDX) models
Conclusion
The interactions between cancer and immune cells are complex and context dependent. Therefore, it is crucial to use the right model to study these interactions to develop new immuno-oncology therapeutics.
At Noble Life Sciences, we offer a portfolio of both xenograft and syngeneic models that our clients may use to assess the in vivo efficacy of their novel CAR T-cells and other cell therapy products. As a result, we can meet a variety of study endpoints to generate high-quality and reproducible data that your studies need. Noble also offers live animal imaging for preclinical studies using PerkinElmer IVIS Lumina LT III to non-invasively monitor and quantify tumor growth longitudinally in tumor-bearing mice following CAR T cell treatment. The IVIS Lumina LT III is designed for in vivo imaging with both fluorescent and bioluminescent reporters that emit from green to near-infrared signals. The field of view allows the imaging of up to five mice or two rats. Animal handling features include a heated stage, gas anesthesia, and ECG monitoring connections. In addition, Noble provides multicolor flow cytometry and Next Generation Sequencing services that can be combined with live animal imaging and immuno-oncology animal models to obtain more robust data for oncology drug development.
